Not many years ago you would have been considered mad to suggest that a virus could trigger cancer, MS or some autism.
We now have
a vaccine to prevent cancers triggered by the human papillomavirus (HPV). Young
people aged 9 - 26 are offered this vaccine in many wealthy countries.
It is
believed that the Epstein-Barr virus (EBV) contributes to about 1.5% of all
cases of human cancer.
EBV causes
mononucleosis (IM, mono), also
known as glandular fever. A commercial vaccine does not yet exist but is
thought to be achievable.
Multiple
sclerosis (MS) has long been thought to have a viral trigger. I have been
reading about impaired myelination for 10 years and it takes a very long time
for ideas to get confirmed. In the case
of MS it is again the Epstein-Barr virus. Almost all adults have been exposed
to this virus and most people do not develop MS. The science suggests that multiple events are
needed to trigger MS, but that a required one is the presence of this virus. That would suggest that if children were
vaccinated against EBV, they could not go on to develop MS later in life.
Epstein-Barr virus may be leading cause of multiple sclerosis
“The hypothesis that EBV causes MS has been investigated by our group and others for several years, but this is the first study providing compelling evidence of causality,” said Alberto Ascherio, professor of epidemiology and nutrition at Harvard Chan School and senior author of the study. “This is a big step because it suggests that most MS cases could be prevented by stopping EBV infection, and that targeting EBV could lead to the discovery of a cure for MS.”
Recall that
MS is one of those diseases that is very much more prevalent in females than
men. It is the opposite of autism.
The science
is moving on and now has got the point of explaining why EBV can cause
cancer. The actual biological pathways
have been proposed.
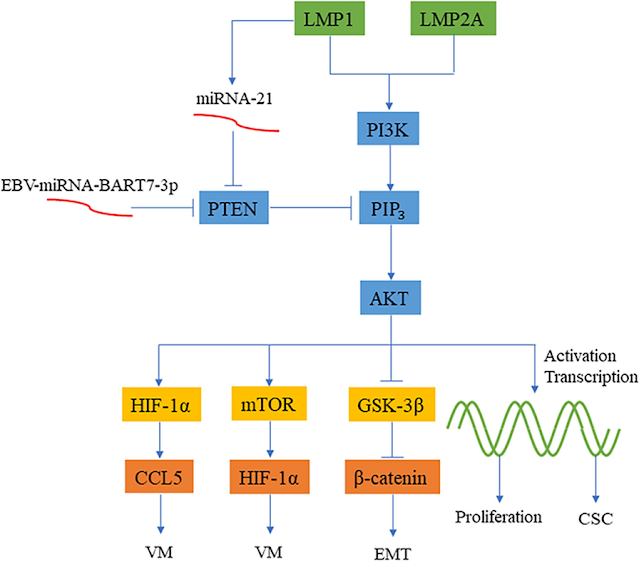
Signaling pathways of EBV-induced oncogenesis
One of several examples is EBV-induced oncogenesis through the
PI3K/AKT signaling pathway.
EBV-induced
oncogenesis through the PI3K/AKT signaling pathway. LMP1 and LMP2A promote
angiogenesis through the PI3K/AKT/HIF-1α/CCL5 signaling axis and the
PI3K/AKT/mTOR/HIF-1α signaling axis, respectively. LMP1 inhibits PTEN through
miRNA-21 and enhances the PI3K/AKT signaling pathway to promote the formation
and proliferation of CSCs. EBV-miRNA-BART7-3P can also promote the high
expression of β-catenin by inhibiting PTEN, leading to EMT
Regular
readers may notice overlaps with what we have seen in autism. The same pathway can lead to autism.
Here is a
recent autism paper on this same pathway.
Targeting
PI3K-AKT/mTOR signaling in the prevention of autism
The role of viruses in autism
Now we have
established that medicine accepts that a virus can play a role in triggering
cancer and that science points a finger at a virus being a trigger for MS, it
is not so crazy to think about the role viruses might play in some autism.
The easy
part – Maternal Immune Activation (MIA)
We have
clear evidence that if a pregnant mother’s immune system is activated during
pregnancy the incidence of autism rises.
In this case it is the immune response that causes the problem, rather
than the specific virus.
Virus
specific
One very
damaging virus, spread by mosquitos, is Zika. If a mother is infected during pregnancy it may lead to microcephaly (small
head), brain damage and joint/muscle malformation in her child.
Endogenous
retroviruses
It has been
suggested in the research for many years that endogenous retroviruses play a
role in autism.
Human
endogenous retroviruses (HERVs) are DNA sequences within human chromosomes;
they comprise 1 to 8% of the human genome.
HERVs represent footprints of previous retroviral infection and have
been termed “fossil viruses”.
Demystified . . . Human endogenous retroviruses
Human endogenous retroviruses (HERVs) are a family of viruses within our genome with similarities to present day exogenous retroviruses. HERVs have been inherited by successive generations and it is possible that some have conferred biological benefits. However, several HERVs have been implicated in certain cancers and autoimmune diseases. This article demystifies these retroviruses by providing an insight into HERVs, their means of classification, and a synopsis of HERVs implicated in cancer and autoimmunity. Furthermore, the biological roles of HERVs are explored.
Take home messages
o
Human
endogenous retroviruses (HERVs) make up part of our genome and represent
footprints of previous retroviral infection
o
HERVs
possess a similar genomic organisation (gag–pol–env) to present day exogenous
retroviruses but are not infectious
o
The
HERV-K superfamily represents one of the most active HERVs and is capable of
producing retroviral particles
o HERVs
may be of benefit to the host but could also be harmful, and may be involved in
certain autoimmune diseases and cancers
I came across this article recently:
Could an Ancient Virus Be a Genetic Driver of
Autism?
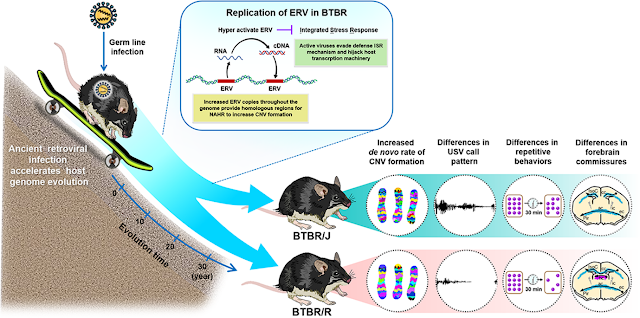
Genome and transcriptome analysis revealed BTBR autism mouse models have increased levels of endogenous retrovirus genes. BTBR/R models of ASD showed differences in the expression of a variety of genes that are indicative of endogenous retrovirus activation. BTBR/R mice exhibit autistic-like behaviors without reduced learning abilities.
Overall, the study revealed that retrovirus activation causes the copy number variants in BTBR mice to increase, which leads to the differences in behavior and brain structure seen in BTBR/J and BTBR/R mice.
Further Developments
BTBR/J mice are widely used by researchers as a mouse model of autism. However, the results of this study highlight the usefulness of the other lineage of BTBR/R mice because they exhibit autistic-like behavior without compromised spatial learning ability. The results also suggest that it may be possible to develop new treatments for autism that suppress ERV activation.
Furthermore, it is necessary to classify autism subtypes
according to their onset mechanism, which is a vital first step towards opening
up new avenues of treatment for autism.
Here is the
full paper, which comes from the RIKEN Brain Science Institute in Japan, which
has been mentioned in a previous post.
The BTBR T+Itpr3tf/J (BTBR/J) strain is one of the most valid models of idiopathic autism, serving as a potent forward genetics tool to dissect the complexity of autism. We found that a sister strain with an intact corpus callosum, BTBR TF/ArtRbrc (BTBR/R), showed more prominent autism core symptoms but moderate ultrasonic communication/normal hippocampus-dependent memory, which may mimic autism in the high functioning spectrum. Intriguingly, disturbed epigenetic silencing mechanism leads to hyperactive endogenous retrovirus (ERV), a mobile genetic element of ancient retroviral infection, which increases de novo copy number variation (CNV) formation in the two BTBR strains. This feature makes the BTBR strain a still evolving multiple-loci model toward higher ASD susceptibility. Furthermore, active ERV, analogous to virus infection, evades the integrated stress response (ISR) of host defense and hijacks the transcriptional machinery during embryonic development in the BTBR strains. These results suggest dual roles of ERV in the pathogenesis of ASD, driving host genome evolution at a long-term scale and managing cellular pathways in response to viral infection, which has immediate effects on embryonic development. The wild-type Draxin expression in BTBR/R also makes this substrain a more precise model to investigate the core etiology of autism without the interference of impaired forebrain bundles as in BTBR/J.
Hyper-activation of ancient retroviral infection accelerates host genome evolution toward ASD susceptibility by increasing the chance of CNV formation. The accumulated genetic variations lead to the divergence of autistic-like behaviors in both BTBR strains. Active ERV also recapitulates the viral infection process of ISR pathway invasion and IRES-mediated translation, which changes the global transcriptome during embryonic development in BTBR mice. BTBR/R has severer core symptoms of autism and wildtype Draxin expression, which suggests BTBR/R is a valid autism model with unaffected forebrain bundles.
There have
been previous studies looking into ERVs and autism.
Human endogenous retroviruses (HERVs) are genetic elements, derived from their exogenous retroviral counterpart by a process of germline infection and proliferation within the human genome, and their integration as proviruses led to the fixation and the vertical transmission, following Mendelian laws. HERVs currently make up ~8% of the genetic material, and some of them have been cooped for physiological functions. Otherwise, their activation in response to environmental factors has been associated with human pathological conditions. In the setting of neurodevelopmental disorders, HERVs have been proposed as contributing factors involved in Autism Spectrum Disorders (ASD), spanning the bridge between genetic susceptibility, environmental risk factors and immune response. We described a distinct expression profile of some HERV families and cytokines in lymphocytes from autistic children and in their mothers suggesting a close mother-child association in ASD. Moreover, in vitro treatment with an antiretroviral drug was able to restore the expression level of HERVs and cytokines providing new insights into the potential role of HERVs as biomarkers of ASD and raising the possibility of using HERVs expression as a therapeutic target for a tailored approach to patient care.
Conclusion
We know that
some cancer is preventable via a vaccine blocking the progress of a virus,
hopefully more types of cancer will be prevented in future.
Some of the
suggested modes of action for the Epstein-Barr virus (EBV) to cause cancer do
involve pathways that are very relevant to autism.
It appears
that an effective EBV vaccine might protect women (and some men) from
developing multiple sclerosis (MS). Will
it also have the effect of reducing their chance of giving birth to a child
with autism? Time will tell.
Any kind of
illness, viral or other, may trigger an exaggerated immune response during
pregnancy and increase the incidence of autism. This is the basis of one of the common animal models of autism, called Maternal Immune Activation (MIA).
The human endogenous
retroviruses (ERVs) accumulated as junk
in our DNA do appear to be able to affect gene expression leading to cancer,
autoimmune disease and indeed some autism.
The Japanese
researchers from RIKEN suggest that it may be possible to develop new
treatments for autism that suppress ERV activation.
One logical
question is whether viruses are relevant just to causing autism or its ongoing
level of severity.
In the case
of cancer and MS it looks like the virus is primarily involved in triggering
the disease. Once the process has started,
the benefit from suppressing the virus may have passed.
Can existing
antiviral drugs treat some autism? Antiviral
drugs work just for specific viruses and they just suppress them, rather than eliminating them.
The antiviral drug Valtrex has long been used by some doctors to treat autism in the US. Just Google it and you will
find enthusiasts like parent Jenny McCarthy -- “when we started him on Valtrex, speech
started pouring out of him”. There have been no clinical trials.
This is an area where more research genuinely is needed. Hopefully the RIKEN Brain Science Institute will translate their ERV findings into approved therapies. That is what is supposed to happen, but usually does not when it comes to autism.
Autism is nowadays such a broadly defined diagnosis, just about anything might have caused it. Autistic behaviors have been caused by a bacterium, a fungus/mold and very possibly a virus. If only it was as straightforward as understanding and treating MS.