Source: https://ojrd.biomedcentral.com/articles/10.1186/s13023-021-01744-1/figures/2
I recently received an email from a
mother in New Zealand asking about what might help her adult son, recently
diagnosed with an extremely rare type of “autism” called elF3f - related neurodevelopmental disorder.
This post is just based on a preliminary
investigation, I think much more would be possible if a serious full-time
review was made. This applies to all the other single gene autisms that are “untreatable”.
eIF3f (eukaryotic translation initiation factor 3 subunit f)
elF3f is one of the more complicated genes/proteins with multiple functions. In
layman’s terms it is involved in making all the other proteins.
eIF3f is a subunit of the eIF3 complex, hence
the “f” on the end. It is required for several steps in the initiation of
protein synthesis.
We saw how elF4 plays a role in how Fragile X
causes intellectual disability. eIF4 is another
translation initiation factor that plays a key role in the initiation of
protein synthesis.
The eIF4 complex and the eIF3 complex interact with each other
to form the translation initiation complex. This complex is responsible for
bringing together the mRNA, the ribosome, and the initiator tRNA, which allows
protein synthesis to begin. I did warn
you it gets complicated!
eIF4 and eIF3 are
both essential for the initiation of protein synthesis.
eIF3f
is also involved in the regulation of cell growth and proliferation, making it
a target gene in cancer therapy, where eIF3f can be overexpressed or under-expressed.
In spite of what the Simon’s
Foundation’s Searchlight project
Simons
Search - Partnering with families. Understanding genetic changes.
Driven
by science. United by hope
In
order to create scientific breakthroughs for rare genetic neurodevelopmental
disorders, families and scientists must come together. Simons Searchlight‘s
mission is to shed light on these disorders by collecting high-quality,
standardized natural history data and building strong partnerships between
researchers, industry and families. Families like yours are the key to making
meaningful progress.
and others say that “at this point, there are no medicines
designed to treat the syndrome”, there certainly are potential treatment
strategies available.
The mother did question whether there
are similarities with Rett syndrome. You
can apparently reduce expression of eIF3f
using the common supplement EGCG (Epigallocatechin Gallate). EGCG has been
found to benefit Rett syndrome.
I think what is likely required for eIF3f-related neurodevelopmental
disorder is the exact opposite, which is to increase expression of eIF3f.
Sources of data:-
GeneCards - EIF3F Gene - Eukaryotic Translation Initiation Factor 3
Subunit F
https://www.genecards.org/cgi-bin/carddisp.pl?gene=EIF3F
RGD - EIF3F (eukaryotic translation
initiation factor 3 subunit F) Homo sapiens
https://rgd.mcw.edu/rgdweb/report/gene/main.html?id=1314535
The above two sites do provide a great deal of information, but I think a lot is auto-generated and there are mistakes.
What we are looking for are safe
substances that change expression of the gene eIF3f.
According to GeneCards there is only
one substance - quercetin.
According to RGD there is a long
list. This did look very promising, but
when I looked at the linked references I did not always find that the
supporting data exists. This is a
problem with AI (artificial intelligence), it can make things up.
Sometimes you have to go back to the
basic science.
There is evidence that activating
the PI3K/AKT/mTOR signaling pathway will increase eIF3f
expression.
One known was to do that would be via increasing IGF-1 – insulin-like
growth factor 1. You can inject IGF-1 and it has even been trialed in autism.
In New Zealand there is an OTC supplement called CGPMax that claims to
increase IGF-1.
I checked and indeed there is some evidence that CGPMax may also increase the expression of
eIF3f.
“There is some evidence that CGPMax may also
increase the expression of eIF3f. In a study of ER-positive breast cancer
cells, CGPMax was shown to increase the expression of eIF3f mRNA and protein.
This was thought to be due to the inhibition of CDK4/6, which led to the
activation of the PI3K/AKT/mTOR signaling pathway.”
AI generated
Since our reader is in New Zealand and wants a supplement rather than a drug, I think CGPMax is a good fit and certainly worth a trial.
One of the substances suggested by the
RGD site was valproic acid. This looked
great news because valproic acid, an anti-epileptic drug (AED), is often used to safely treat even young
children.
Why does Valproic acid apparently
increase eIF3f mRNA? That would highly likely be down to it being
an HDAC inhibitor which causes it to make epigenetic changes that turn on/off
our genes.
We know that some single gene autism
can be treated by HDAC inhibitors, at least in mouse models. The potent HDAC
inhibitors are now used to treat cancer. One parent I met at the Thinking
Autism conference was desperate to access one of these potent drugs for her
child’s single gene autism, similar to Kabuki syndrome.
Broccoli sprouts produce an HDAC
inhibitor, called sulforaphane.
I could not find any supporting data
why valproic acid was listed, the linked reference did not actually refer to eIF3f.
Nonetheless it is harmless to try
broccoli sprouts.
Quercetin
Another common product popped up in my
brief review and that was Quercetin. I had not expected to find that. There is
a reaction between quercetin and eIF3f.
It is not fully understood.
Quercetin is a widely available OTC
product and simple to trial.
Estradiol
It is
known that estradiol can increase the expression of eIF3f.
The
effect of estradiol on eIF3f expression is likely mediated by the estrogen
receptor alpha (ERα). We have seen that
estrogen receptor beta (ERβ) is under-expressed in autism.
Increasing estradiol, or indeed
reducing testosterone, has been proposed as an autism therapy. This is not a
simple strategy. In cancer therapy
radical steps are taken to reduce sex hormones, because it is the only way to
stop the growth of certain types of cancer.
Disturbing the level of male/female
hormones will have body-wide effects. The
“men” who currently take large doses of female hormones are going to have
consequences later in life.
There is dietary therapy in the form
of phytoestrogens that is known to be safe.
The Japanese eat a lot of soy products.
Soy is a particularly good source of
phytoestrogens, especially a type of phytoestrogen called isoflavones.
Isoflavones are similar in structure to estrogen, but they are much weaker.
Incorporating more soy products into
diet would seem a reasonable strategy.
Others
There is some evidence that the antibiotic gentamicin can activate the
gene eIF3f. It is given by injection.
Among the list of substance that can
increase eIF3f mRNA are
some quite toxic substances like BPA found in plastic packaging. Another interesting option was listed under “anti-rheumatic drugs”, this actually
refers to tocilizumab. This is an anti-arthritis drug given to people over the
age of two. Since it ends in -mab, we
can infer that it contains monoclonal antibodies, in this case to
interleukin-6.
Tocilizumab
would likely be helpful in many people with other kinds of autism with a strong
auto-immune component.
eIF3f-specific treatments
vs treat as idiopathic autism
We know from readers with children
with different single gene autisms, that are supposed to be untreatable, that
these children often respond well to therapies in use for autism of unknown
origin (idiopathic autism).
Almost all autism features
neuroinflammation, activated microglia etc. Most autism features oxidative
stress. Most autism features impaired
myelination. Much autism features mitochondrial dysfunction.
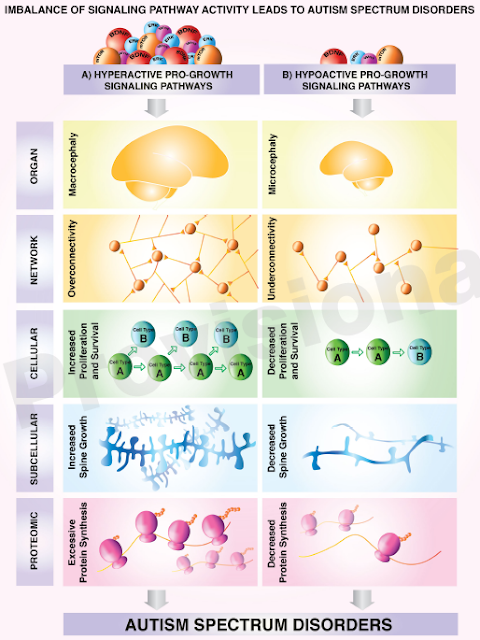
There are specific insights that a
genetic diagnosis does give you. In the
case of eIF3f, we are
dealing with hypo-active (REDUCED) pro-growth signaling. That means the
opposite to the kids born with macrocephaly (big heads).
This excellent framework was explained in this old post
https://www.epiphanyasd.com/2015/12/one-of-thousands-autism.html
IGF-1 was mentioned earlier as a possible therapy. Note that growth hormone (GH) is made in the anterior pituitary gland, it is released into the blood stream, and then stimulates the liver to produce IGF-1. IGF-1 then stimulates systemic body growth, and has growth-promoting effects on almost every cell in the body.
More IGF-1 would lead to more growth.
Even in an adult you can increase the density of dendritic spines.
As shown in the chart above on the
lower right, in today’s disorder we have decreased protein synthesis.
Now back to the science and the basics of this syndrome.
eIF3f-related
neurodevelopmental disorder (EIF3F-RND) is a rare genetic disorder that causes
a variety of neurological and developmental problems. It is caused by mutations
in the eIF3f gene,
which provides instructions for making a protein that is involved in protein
synthesis. It has to be inherited from both parents.
If both parents are carriers, there is a 25% chance
that each child will have EIF3F-RND, a 50% chance that each child will be a
carrier, and a 25% chance that each child will not have EIF3F-RND and will not
be a carrier.
If only one parent is a carrier of the mutated gene, there is a 50% chance that each child will be a carrier, and a 50% chance that each child will not be a carrier and will not have EIF3F-RND.
The incidence of EIF3F-related neurodevelopmental disorder (EIF3F-RND) is
unknown. However, it is estimated to be a very rare disorder, affecting less
than 1 in 100,000 people. This is likely due to the fact that EIF3F-RND is
caused by mutations in a single gene. In order for a child to be affected, both
parents must carry a copy of the mutated gene. If only one parent is a carrier,
the child will be a carrier, but will not be affected.
The incidence of EIF3F-RND may also be underestimated, as it is a relatively newly identified disorder. As more people are diagnosed with the disorder, the incidence rate may increase.
EIF3F-RND is caused by under-expression of the eIF3f protein.
Symptoms of
EIF3F-RND can vary widely from person to person, but may include:
- Intellectual disability
- Developmental delay
- Seizures
- Hypotonia (low muscle tone)
- Microcephaly (small head size)
- Autism spectrum disorder
- Facial dysmorphism
Source:
https://ojrd.biomedcentral.com/articles/10.1186/s13023-021-01744-1/figures/2
Interactions with other genes/proteins
One feature of the GeneCards website
is that you can see a representation of which are the most important
interactions of a gene/protein.
This can sometimes suggest a possible
therapy, since one of these related genes might be easier to treat.
In the case of eIF3f almost all the interactions are with other
elF-somethings.
The
RPS-somethings below are all genes that translate mRNA into proteins.
So, everything below is part of the machinery cells have to make proteins.
EIF3F-related neurodevelopmental
disorder research
The EIF3F-NDR research is still in its infancy.
There need to be a models made that can suggest which downstream genes
are affected and hence might be treatable.
An eIF3f activator is a drug or other compound that can increase the expression or
activity of the eIF3f protein.
Currently, there are no known eIF3f activators that are approved for clinical use. However, researchers are
developing a number of different approaches to activating EIF3F, including:
- Small
molecule drugs: Researchers are screening libraries of small
molecules to identify compounds that can bind to eIF3f and increase its
activity.
- Gene
therapy: Gene therapy could be used to deliver a working copy of the eIF3f gene to cells in the nervous system.
- CRISPR
gene editing: CRISPR gene editing could be used to correct mutations
in the eIF3f gene.
In addition to the above
approaches, there are a number of other things that could potentially be done
to activate eIF3f, such as:
- Identifying
and targeting upstream regulators of eIF3f: Researchers could
identify and target other proteins or genes that regulate the expression
or activity of eIF3f. This could lead to the development of new drugs or
other therapies that could be used to activate eIF3f indirectly.
- Understanding
the role of eIF3f in different cell types: Researchers are still
learning about the role of eIF3f in different cell types in the nervous
system. This knowledge could be used to develop targeted therapies that
activate eIF3f in the specific cell types where it is needed most.
Background
An identical homozygous missense variant in EIF3F,
identified through a large-scale genome-wide sequencing approach, was reported
as causative in nine individuals with a neurodevelopmental disorder,
characterized by variable intellectual disability, epilepsy, behavioral
problems and sensorineural hearing-loss. To refine the phenotypic and molecular
spectrum of EIF3F-related neurodevelopmental disorder, we examined
independent patients.
Results
21 patients were homozygous and one compound
heterozygous for c.694T>G/p.(Phe232Val) in EIF3F. Haplotype
analyses in 15 families suggested that c.694T>G/p.(Phe232Val) was a founder
variant. All affected individuals had developmental delays including delayed
speech development. About half of the affected individuals had behavioral
problems, altered muscular tone, hearing loss, and short stature. Moreover, this study suggests that
microcephaly, reduced sensitivity to pain, cleft lip/palate, gastrointestinal symptoms
and ophthalmological symptoms are part of the phenotypic spectrum. Minor
dysmorphic features were observed, although neither the individuals’ facial nor
general appearance were obviously distinctive. Symptoms in the compound
heterozygous individual with an additional truncating variant were at the
severe end of the spectrum in regard to motor milestones, speech delay, organic
problems and pre- and postnatal growth of body and head, suggesting some
genotype–phenotype correlation.
Conclusions
Our study refines the phenotypic and expands the
molecular spectrum of EIF3F-related syndromic neurodevelopmental
disorder.
The cancer research
Cancer research is much more advanced
and better funded than autism research.
As you can see in the table below,
decreased expression of eIF3f is
feature of several common cancers. If you can upregulate eIF3f you might have a
viable cancer therapy.
As in
many types of autism, the potential exists to repurpose cancer drugs as and
when they get developed and approved. HDAC inhibition is perhaps the best
example. So far people are too scared to try the new potent HDAC inhibitors in
human single-gene (monogenic) autism.
https://theses.hal.science/tel-01679873/document
Alternatively, an indirect regulation of the activity of eIF3 is performed by association of its subunits with other proteins involved in the regulation of protein synthesis. For example, the subunit eIF3e binds p56 in interferon-treated or virus-infected mammalian cells, and inhibits the translation in vitro and in vivo [43, 44]. The subunit eIF3g interacts with Paip1, a Poly (A)-binding protein and stimulates translation initiation [45] whereas the subunits eIF3h and eIF3f interact with TRC8, a ubiquitin E3 ligase, and inhibit protein synthesis, possibly through ubiquitilation of eIF3 or some other translational components [46]. These mechanisms and interacting partners render eIF3 a pivotal player in controlling the protein synthesis and degradation.
All
these data confirm that eIF3f has a multileveled control of multiple functions
in the cells, outside its usual function in translation. Keeping it in mind,
targeting eIF3f may be a strategy to reorganize different intracellular
pathways and alter the basis of the balance between cell proliferation and
apoptosis. Thus, eIF3f
represents a lead candidate to use for biotherapeutic applications both for
inhibiting the growth of cancer cells or muscle atrophy and thus
preventing its progression into irreversible cachexia.
Conclusion
Personally, I would treat EIF3F-NDR
with two parallel approaches:
· As idiopathic autism with hypo-active pro-growth sigaling autism (small heads/microcephaly)
· Gene specific with clever ideas targeting the effects of eIF3f under-expression.
Is the cognitive impairment responding
to bumetanide? In the models of Rett
syndrome and Fragile-X this is the case. For EIF3F-NDR you could just make your
own trial.
For sure there will be oxidative
stress in EIF3F-NDR due to the malfunctioning in the protein synthesis
“machinery”. NAC is the antioxidant of
choice and is OTC.
EIF3F-NDR can be associated with GI
dysfunction, as is much of broader autism.
When treated this often leads to improvements in behavior.
Increasing IGF-1 looks achievable.
Nerve growth factor (NGF) may be
upregulated by Lion’s Mane mushrooms, according to the research.
BDNF (brain derived neurotropic
factor) can be up regulated. Certain foods and nutrients have been shown to
increase BDNF levels. For example, one study found that lutein supplementation
increased BDNF levels in the blood. Other foods and nutrients that have been
shown to increase BDNF levels include omega-3 fatty acids, magnesium, and zinc. Some drugs increase BDNF such as lithium,
SSRIs, modafinil. Statins such as Simvastatin and Atorvastatin are known to
increase BDNF.