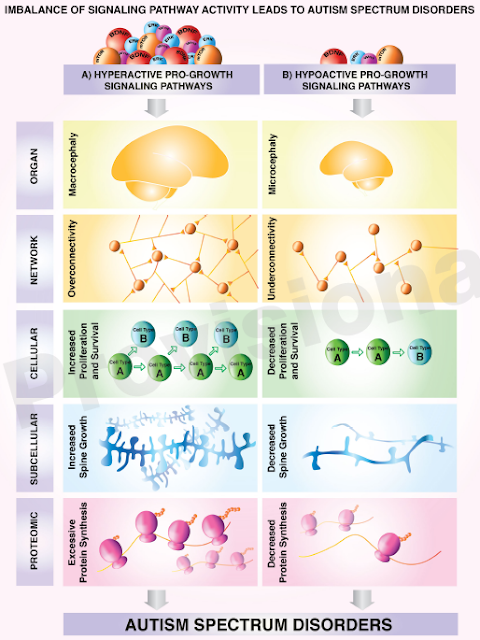
Source: https://www.cell.com/neuron/pdf/S0896-6273(21)00466-9.pdf
Today’s post is drawn from a workshop I am invited to present at an autism conference in Abu Dhabi.
I decided to talk about advances in personalized medicine – no surprise there. Since I have 2 ½ hours, I thought I will need some interesting examples to maintain the audiences interest. One such topic is going to be Rett syndrome.
I regard Rett syndrome and all the other such syndromes in this blog as “single gene autisms” (monogenic autism). If you apply the American DSM classification, from 2013 onwards Rett syndrome is no longer part of autism. Hopefully there are no such purists attending in Abu Dhabi.
Two gene therapies for Rett syndrome are currently undergoing human trials and one drug therapy has been FDA approved. This looks very encouraging, so let’s dig a little deeper.
Rett syndrome can present with a wide range of disability ranging from mild to severe.
Rett syndrome is the second most common cause of severe intellectual disability after Down syndrome.
Other symptoms may include:
• Loss of speech
• Loss of purposeful use of hands
• Loss of mobility or gait disturbances
• Loss of muscle tone
• Seizures or Rett “episodes”
• Scoliosis
• Breathing issues
• Sleep disturbances
• Slowed rate of growth for head, feet and hands
Here are the new therapies:
TSHA-102: This gene therapy, developed by Taysha Therapeutics, is a gene replacement therapy that aims to deliver a functional copy of the MECP2 gene to brain cells. It utilizes an AAV-9 virus to carry the miniMECP2 gene product into cells for the body to produce more MeCP2 protein, which is deficient in Rett syndrome. As of February 2024, Taysha completed dosing for the first cohort (low dose) in their REVEAL Phase 1/2 adolescent and adult trial in Canada, with positive interim data on safety. They are also conducting trials in the US for both pediatric and adolescent/adult populations.
NGN-401: This gene therapy, by Neurogene Inc., employs a different approach. It uses an AAV9 vector to deliver a regulated version of the MECP2 gene called EXACT. This technology aims to control the amount of MECP2 protein produced by the gene, mitigating the risk of overproduction. NGN-401 is currently in a Phase 1/2 trial for girls with Rett syndrome aged 4 to 10 years old.
Daybue (trofinetide)
Daybue is the first and only FDA-approved treatment specifically for Rett syndrome in adults and children two years of age and older. It is not a gene therapy, but rather a medication taken orally.
The optimistic AI generated view:
Here's a breakdown of Daybue for Rett syndrome:
- Mechanism: The exact way Daybue works in Rett syndrome isn't fully understood, but it's believed to target neuroinflammation and support synaptic function.
- Dosage: The recommended dose is based on the patient's weight and is taken twice daily, morning and evening, with or without food.
- Administration: Daybue comes as an oral solution and can be taken directly or through a gastrostomy tube if swallowing is difficult.
- Efficacy: Studies have shown that Daybue can improve symptoms of Rett syndrome, including reducing scores on the Rett Syndrome Behavior Questionnaire (RSBQ) and showing improvement on the Clinical Global Impression-Improvement (CGI-I) scale.
- Side Effects: The most common side effects of Daybue are diarrhea and vomiting. Weight loss can also occur in some patients. It's important to consult with a healthcare professional for monitoring and managing any potential side effects.
Daybue is an expensive medication. Here's what we know about the cost:
- List Price: The list price of Daybue is around $21.10 per milliliter.
- Annual Cost: This translates to an estimated average annual cost of around $375,000 for patients.
- Dosage Variability: It's important to note that the dosage of Daybue is based on a patient's weight, so the annual cost can vary depending on the individual.
Insurance and Assistance Programs:
- The high cost of Daybue highlights the importance of insurance coverage. Whether insurance covers Daybue and to what extent will depend on your specific plan.
- The manufacturer, Acadia Pharmaceuticals, offers a copay program called Daybue Acadia Connect. This program may help eligible commercially insured patients pay $0 for their monthly prescription.
What are the parents' groups saying?
Not as good as you might be expecting for $375,000 a year.
Affordable potential alternatives to Daybue/Trofinetide
Daybue/Trofinetide is the product of decades of research into a growth factor called IGF-1.
It is a complicated subject and as usual the abbreviations can be confusing.
As you will see below there already is an OTC product commercialized by one of the original researchers, Dr Jian Guan.
One Rett syndrome parent, who reads this blog, has trialed cGP and sees a benefit. You rather wonder why the Phelan-McDermid, Pitt Hopkins, Angelman and Prader-Willi parents don’t follow him and splash out 50 USD and make a trial.
Gene-therapy
Gene therapy is undoubtedly very clever and ultimately will likely be the best therapy. It still may not be that silver bullet.
To be effective gene therapy needs to be given at a very young age, ideally as a fetal therapy prior to birth. Note that we saw that in the Rett mouse model they gave bumetanide to the pregnant mother just before birth.
Fetal therapy is not a crazy idea and much is already written about it; many pregnancies are terminated because genetic anomalies are detected prior to birth. Down syndrome is the best-known example. Fetal therapy is realistic for some disorders.
Girls with Rett syndrome are often diagnosed first with idiopathic autism and then years later with a more precise diagnosis of Rett syndrome. This is a common experience among readers of this blog.
Classic Rett syndrome
The average age of diagnosis for this form is around 2.5 years old in the US and 5 years old in the UK. Why do you think that is?
Research in mouse models has shown that the effect of gene therapy ranges from curative when given extremely young to more limited the later it is given.
Off-target effects
Gene therapy has the potential for off-target effects. This is a significant concern in the field and researchers are actively working on ways to minimize these risks. Here is a breakdown of what off-target effects are and why they matter:
During gene therapy, a modified gene is delivered to target cells with the aim of correcting a genetic defect.
Ideally, the modified gene integrates into the intended location in the genome.
However, there's a chance it might insert itself into unintended locations (off-target sites).
Potential Consequences of Off-Target Effects
Disrupting normal genes at off-target sites could lead to unpredictable and potentially harmful consequences. This could include triggering uncontrolled cell growth, which is a risk factor for cancer.
It can also cause unexpected side effects depending on which genes are accidentally disrupted.
Minimizing Off-Target Effects
Researchers are developing various strategies to improve the accuracy and specificity of gene therapy techniques.
This includes using more precise gene editing tools like CRISPR-Cas9 with optimized guide RNAs to reduce off-target edits.
Additionally, researchers are working on methods to detect and potentially repair any off-target modifications that might occur.
Over-expression of the target gene
Yes, there is a possibility that the replaced gene in gene therapy could overproduce the expressed protein. This can be a potential complication and researchers are working on ways to control the level of protein expression. Here's a breakdown of the concern:
- Gene Dosing: Ideally, gene therapy aims to deliver a functional copy of the gene at the right amount to compensate for the deficiency.
- Overproduction Risks: However, if the delivered gene is too active or multiple copies are inserted, it can lead to overproduction of the protein.
Consequences of Protein Overproduction:
- Overproduction of a protein can disrupt the delicate balance in the cell, potentially leading to cell dysfunction or even cell death.
- In some cases, the protein itself might have harmful effects if present in excessive amounts.
Controlling Protein Expression:
Researchers are developing several strategies to control protein expression in gene therapy:- Promoter selection: Using promoters that have a weaker switch can help regulate protein production.
- Viral vectors: Engineering viral vectors to control the number of gene copies delivered to cells.
- Inducible systems: Developing gene therapy methods where the expression of the introduced gene can be turned on and off as needed.
The cost of gene therapy
• Despite the high cost, gene therapy can be a cost-effective treatment for some diseases. This is because it can eliminate the need for lifelong treatment with other medications.
• Here are some examples of the cost of currently available pediatric gene therapies:
• Luxturna (gene therapy for Leber congenital amaurosis type 10): $425,000
• Zolgensma (gene therapy for spinal muscular atrophy type 1): $2.1 million
• Skysona (gene therapy for adrenoleukodystrophy): $3 million
Piperine to correct KCC2 expression in Rett syndrome?
One key feature of Rett syndrome is impaired cognition.
As regular readers are aware, there are many types of treatable intellectual disability (ID).
One type of treatable ID is caused when the GABA developmental switch fails to occur shortly after birth. This creates an excitatory/inhibitory imbalance in neurons which impairs cognition and lowers IQ.
The faulty GABA switch is a feature of many types of autism, but far from all of them.
By using pharmaceuticals to lower chloride within neurons, you can compensate for the failure of the GABA switch.
This treatment can be achieved by:
1. Blocking or down regulating NKCC1
2. Up regulating KCC2
In the paper below they look at up regulating KCC2
Rett syndrome (RTT) is a neurodevelopmental disorder caused by mutations in the methyl CpG binding protein 2 (MECP2) gene. There are currently no approved treatments for RTT. The expression of K+/Cl− cotransporter 2 (KCC2), a neuron-specific protein, has been found to be reduced in human RTT neurons and in RTT mouse models, suggesting that KCC2 might play a role in the pathophysiology of RTT.
Injection of KEEC KW-2449 or piperine in Mecp2 mutant mice ameliorated disease-associated respiratory and locomotion phenotypes. The small-molecule compounds described in our study may have therapeutic effects not only in RTT but also in other neurological disorders involving dysregulation of KCC2.
Thus, our data demonstrate that activation of the SIRT1 pathway or the TRPV1 channel enhances KCC2 expression in RTT human neurons.
Treatment with piperine (10 μM), an activator of the TRPV1 channel (51), induced a significant rise in KCC2 expression in cultured human neurons
We already knew this was likely from earlier research from Ben Ari, see below for a reminder. Is Piperine an interesting option for those restricted to OTC interventions?
Genetic mutations of the Methyl-CpG-binding protein-2 (MECP2) gene underlie Rett syndrome (RTT). Developmental processes are often considered to be irrelevant in RTT pathogenesis but neuronal activity at birth has not been recorded. We report that the GABA developmental shift at birth is abolished in CA3 pyramidal neurons of Mecp2-/y mice and the glutamatergic/GABAergic postsynaptic currents (PSCs) ratio is increased. Two weeks later, GABA exerts strong excitatory actions, the glutamatergic/GABAergic PSCs ratio is enhanced, hyper-synchronized activity is present and metabotropic long-term depression (LTD) is impacted. One day before delivery, maternal administration of the NKCC1 chloride importer antagonist bumetanide restored these parameters but not respiratory or weight deficits, nor the onset of mortality. Results suggest that birth is a critical period in RTT with important alterations that can be attenuated by bumetanide raising the possibility of early treatment of the disorder.
One day before delivery, maternal administration of the NKCC1 chloride importer antagonist bumetanide restored these parameters but not respiratory or weight deficits, nor the onset of mortality. Results suggest that birth is a critical period in RTT with important alterations that can be attenuated by bumetanide raising the possibility of early treatment of the disorder.
Treating the mother prior to delivery with bumetanide was a partially effective therapy in the mouse model of Rett syndrome.
Piperine
Bumetanide is cheap and very possibly effective in human Rett syndrome, but it is a prescription drug.
Piperine is an OTC supplement and a compound found in black pepper. By activating the TRPV1 channel it causes an increase in expression of the KCC2 transporter that allows flow of chloride out of neurons. So piperine should lower chloride inside neurons. Piperine can cross the blood brain barrier, so when taken orally it should have some effect on intracellular chloride.
Piperine is also a positive allosteric modulator of GABAA receptors
This means that piperine multiplies the effect of whatever GABA is around. This means that in typical people piperine should have anti-anxiety effects.
Piperine was recently found to interact with a previously unknown benzodiazepine-independent binding site.
Researchers are currently toying with the piperine molecule to try and separate the effect on TRPV1 from the effects on GABAA. They want to create 2 new drugs.
1. a selective TRPV1 activator
2. a selective GABAA modulator (PAM)
Piperine as an alternative or complement to Bumetanide?
One effect of piperine would be great to have (TRPV1 activator) but the second effect would not be helpful (positive allosteric modulator of GABAA).
The question is what is the net effect. Nobody will be able to answer that without a human trial.
I was advised long ago by one drug developer than it is best to focus on reducing flow into neurons via NKCC1, rather than increase its exit by KCC2, because nobody had yet been successful with KCC2; many have tried. KCC2 plays a key role in neuropathic pain and that is why it has been researched.
Conclusion
We did see years ago that taking coffee with your bumetanide made sense. Coffee contains compounds that are OAT3 inhibitors and slow down the excretion of bumetanide from the body; coffee increases the effect of bumetanide. You can achieve something very similar by just increasing the dose of bumetanide.
Taking black pepper (piperine) with your bumetanide might be good, or might not be. It certainly would be easy to find out. As with Daybue/Trofinetide, the result is likely to vary from person to person. If GABA function, post- bumetanide, is still a bit excitatory amplifying GABA signaling will make autism worse. If GABA function has been shifted to inhibitory then amplifying GABA signaling will be calming.
Gene therapy will require much earlier diagnosis of single gene autisms.
“Precision medicine” therapies like Daybue/Trofinetide may not be that precise after all and large variations exist in the response, even among children with the same affected gene.
The huge expense means that for most of the world they will see no benefit from gene therapy or indeed “precision medicine.”
The low hanging fruit is to repurpose affordable existing drugs and get the benefit from their secondary effects. This is what I term personalized medicine.
The research clearly indicates that some girls with Rett syndrome likely will benefit from Bumetanide therapy. For a young child this therapy would cost 50 US dollars/euros a year, if you pay the actual price for generics.
Why are they trialing genetic therapies for Rett instead of first doing the obvious thing and trialing cheap bumetanide? They will likely be able to sell the gene therapy for $2 million a shot. There is little interest in trialing a $50 a year therapy.
Our new reader from Turkey, MÜCADELECI ANNE DENIZ ( = FIGHTING MOTHER DENIZ), likely does not have $2 million to spend, but seems to be on the way to creating her own personalized medicine therapy for her son. Good luck to her.
As to the cGP Max supplement, it seems to work for some and have no effect in others. Nobody has reported any side effects. It looks worth a try for Rett syndrome. As a supplement it is not cheap, that is until you see what they charge for Daybue.